Abstract
Treatment guidelines for older patients with severe aplastic anaemia (SAA) are incompletely developed. In particular, there is a paucity of data relating to outcomes among older patients with SAA who receive hematopoietic stem cell transplantation (SCT) from HLA-matched sibling (MSD) or unrelated donors (MUD). SCT is usually performed as second line treatment and it has been postulated that with improvements in conditioning protocols and supportive care, results in older patients would have improved over time. We analysed transplant outcomes in 538 patients aged 50 years and older with SAA who were transplanted between 2005 and 2016.
275 patients (51%) received grafts from MSD, 224 (42%) from 8/8 HLA-matched MUD and 39 (7%) from 7/8 HLA-matched MUD. Median age at SCT was 58 years (range 50-77yrs,), of whom 347 were aged 50-59 years, 174 were aged 60-69yrs and 17 were aged ≥70yrs. 205 patients (38%) reported a performance score less than 90% and the median time from diagnosis to SCT was 10 months. Cyclophosphamide (Cy) with ATG or Cy with fludarabine and ATG were the predominant conditioning regimens for MSD SCT, whereas for MUD SCT, Cy with TBI 200 cGy and ATG or Cy, TBI 200 cGy, fludarabine with ATG were mostly used. 53% of the patients received bone marrow graft and 75% received calcineurin inhibitor (CNI) containing GVHD prophylaxis; 9% received alemtuzumab as part of GVHD prophylaxis.
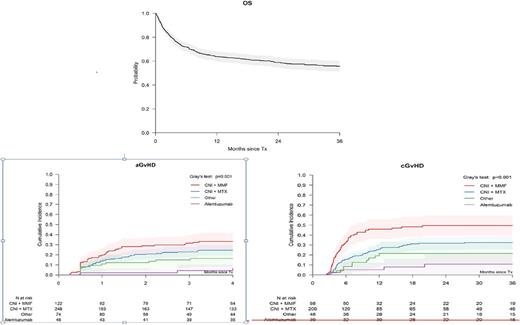
The day-28 cumulative incidence of neutrophil recovery was 84% (95% CI 81-87) and did not differ by patient age. Overall survival (OS) at 3 years for the entire cohort at 3 years was 56% (95% CI 51-60%) (Figure 1). Multivariate analysis confirmed higher mortality for patients with performance score less than 90% (HR 1.37, 95% CI 1.02-1.82; p=0.04) or who received a MUD SCT (HR 1.48, 95% CI 1.02-2.13, p=0.04). No other patient, disease or transplant characteristic was associated with survival. There were no significant differences in mortality risks for patients aged 65-78 versus those aged 50-64 years (HR1.20, 95% CI 0.08-1.74, p=0.36). However, age was significantly associated with grade II-IV acute GVHD; patients aged 65-78 years were at higher risk (HR 1.80, 95% CI 1.18-2.77, p=0.007). On univariate analysis, stem cell source (bone marrow versus peripheral blood) had no impact on acute (p=0.47) or chronic GVHD (p=0.12). Chronic GVHD risks were lower with CNI plus methotrexate (MTX) (HR 0.52, 95% CI 0.34-0.79, p =0.002) and alemtuzumab containing regimens (HR 0.25, 95% CI 0.13-0.48, p,0.001) than with CNI plus mycophenolate (MMF) GVHD prophylaxis. The group of patients who received alemtuzumab had the lowest rate of acute GVHD: 4% (95% CI: 0-10%) compared with 33% (95% CI: 25-42%) with CNI+MMF, 24% (95% CI: 19-30%) with CNI+MTX and 16% (95% CI:8-25%) with other GVHD prophylaxis (non-CNI containing regimens) (p=0.001). The corresponding 2-year rates of chronic GVHD were 11% (95% CI: 1-21%) with alemtuzumab, 50% (95% CI: 40-60%) with CNI+MMF, 32% (95% CI: 26-39%) with CNI+MTX and 21% (95% CI:10-33%) with other GVHD prophylaxis (p<0.001) (Figure 2). Infection was the predominant cause of death accountiing for 40% of deaths.
These data support the feasibility of MSD or MUD SCT in SAA patients aged 50 years and older. However, the data need to be interpreted with caution since the selection of patients very likely has been biased. The importance of the performance status for outcome only underscores that concern. Acute and chronic GVHD adds to the burden of morbidity. Alemtuzumab-containing regimens deserve further study in this patient population.
Petersen: Sanofi: Membership on an entity's Board of Directors or advisory committees. Hallek: Mundipharma: Consultancy, Honoraria, Research Funding; F. Hoffmann-LaRoche: Consultancy, Honoraria, Research Funding; Janssen-Cilag: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding. Potter: Jazz: Honoraria; Pfizer: Other: Advisory board. Peffault De Latour: Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria, Research Funding; Amgen: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.